- Nanoparticles through airway mucus
- Hydrogel-liquid interfaces
- Epithelial tissue morphogenesis
- Interfacial dynamics in complex fluids
- Cell mechanics, chemotaxis and collective migration
- Tear film breakup in healthy and infected eyes
(in collaboration with Leah Keshet, Claude Verdier, and Don Sin)
Cell motility raises many interesting and challenging questions, both mechanical and biological. In chemotaxis, the cell responds to a chemical attractant by initiating biochemical reactions inside, leading to its polarization. At the cell front, high level of certain signaling proteins (e.g. Rac) triggers polymerization of actin filaments and causes protrusion of lamellipodia. At the rear, Rho and myosin activation produces contraction. As an example, the well-known movie below shows a neutrophil chasing down a bacterium. We have built a chemico-mechanical model (full text) for single-cell motility by integrating the biochemistry responsible for polarization and the solid-fluid mechanics underlying the deformation and movement.
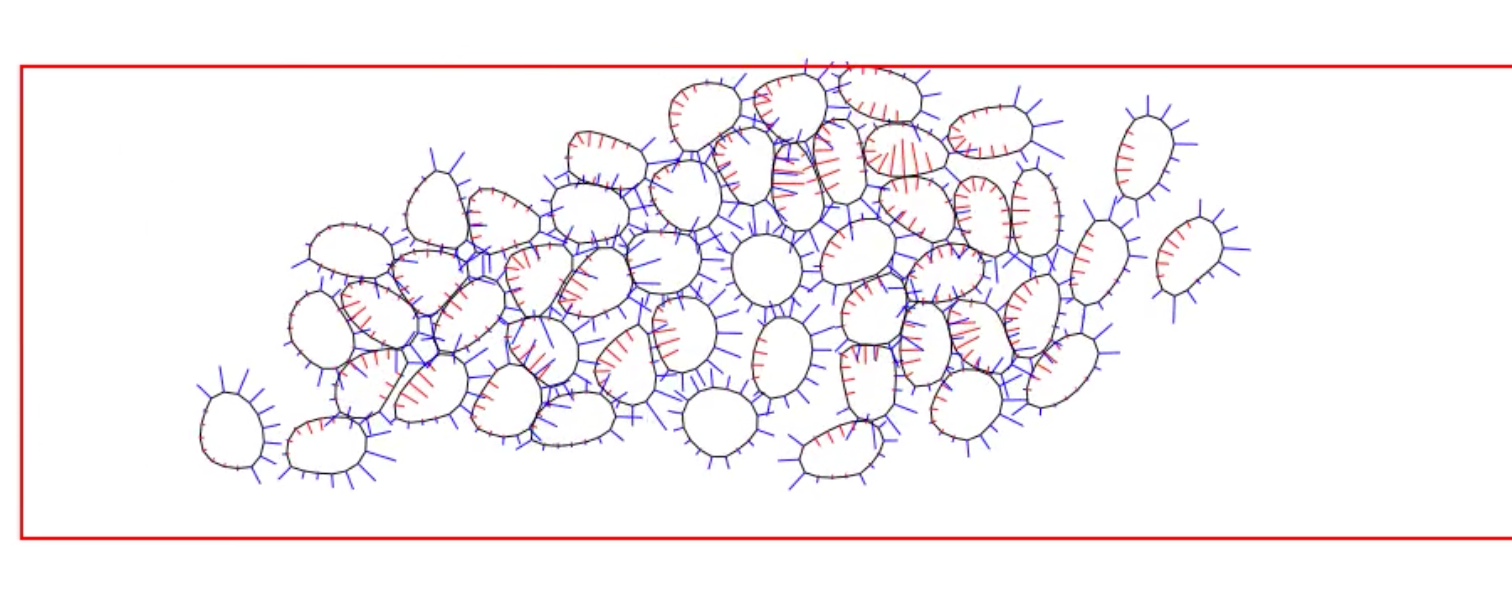
An even more intriguing situation arises when a cluster of cells execute collective migration, which plays prominent roles in a variety of biological processes ranging from wound healing to neural crest cell migration during embryonic development. How do the cells interact among themselves and coordinate their movement? What signaling proteins and pathways are involved, and how do the chemical signaling interact with mechanotransduction? We have proposed a hypothesis of collective migration as an emergent behavior based on contact inhibition of locomotion and co-attraction, and used it to explain the spontaneous migration of a cell cluster in a confined geometry and the group advantage in chemotaxis. The movie below illustrates a simulation of spontaneous collective migration in a confined corridor:
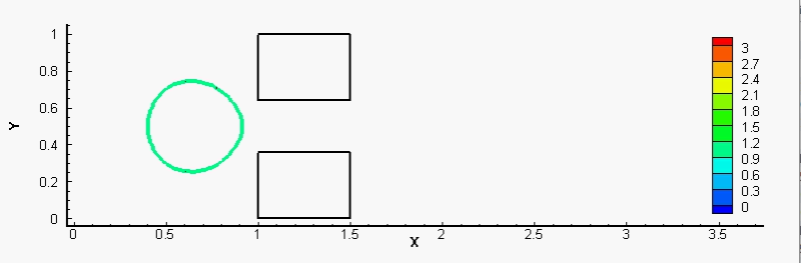
We have also proposed mechanical models to show how cancer cells burrow out of the blood vessel in metastasis, and how white blood cells accomplish a similar act during immune response. More generally, we are interested in the reaction of cells to mechanical forcing and strain. For example, stretch and compression can induce cell fluidization, and neutrophils entering a small microfluidic channel can polarize and activate following fluidization. Accounting for the inhibition of Rac activation by cortical tension, we have modeled Rac growth and potential polarization by a wave-pinning mechanism after the cytoskeleton is fluidized by mechanical strain (full text). The movie below shows a typical simulation of the fluidization of a neutrophil upon entering a narrow channel, and its subsequent activation after exiting.
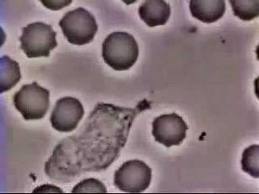
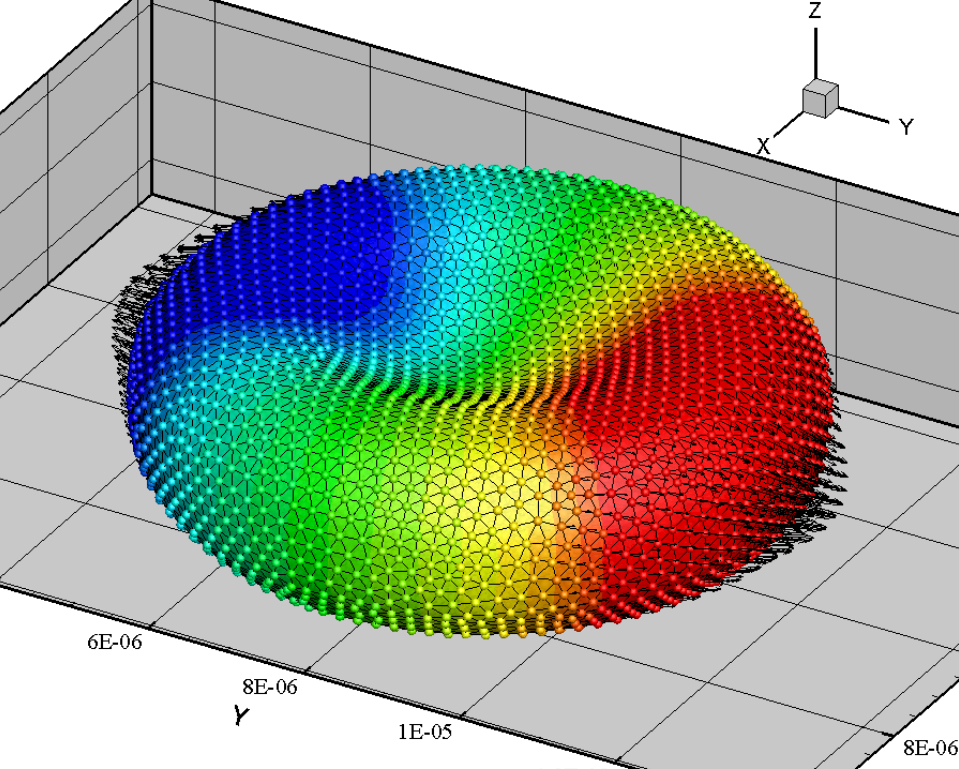
In another line of work, we used particle-based methods to investigate the gradual loss of red-blood-cell (RBC) deformability as a result of malaria infection. The growth of the malaria parasite turns out to play as important a role as the cell membrane rigidification. We have predicted how infected RBC loses its stretchability and eventually blocks capillaries and microfluidic channels. The movie below shows RBC stretching by an optical tweezer simulated by Smoothed Particle Hydrodynamics.