- Organ-on-chip devices
- Nanoparticles through airway mucus
- Hydrogel-liquid interfacial dynamics
- Epithelial tissue morphogenesis
- Complex fluids and interfaces
(in collaboration with UBC colleagues Sarah Hedtrich and Don Sin, and with the group of Mian Long at the Chinese Academy of Science.)
There has been intensifying research to develop complex in vitro cell and tissue models that replicate certain aspects of human organ function. Such organ-on-chip (OoC) devices emulate organ-level (patho)physiology and are currently the most promising human-based approach in biomedical research.
Our work in this area has been motivated by the prospect of using OoCs to improve clinical translation and reduce attrition rate in the drug development process, potentially replacing animal testing. In collaboration with experimentalists, we build mathematical and computational models for OoCs, and use in silico simulations to guide the design and optimization of in vitro devices.
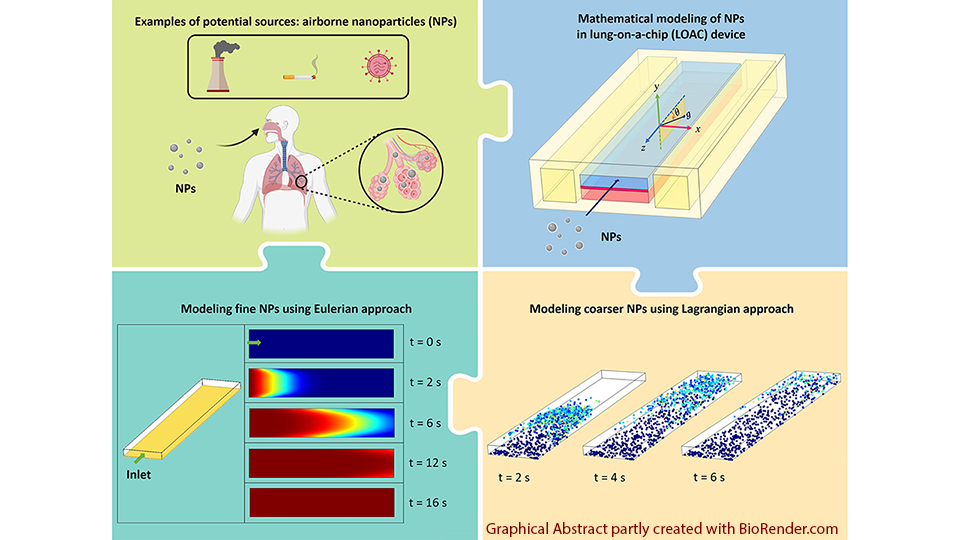
In a Mitacs project, we developed a computational model for the transport and deposition of air-borne nanoparticles in a lung-on-a-chip device; see image above. We have also developed a similarity scaling approach for OoC devices and multi-organ-on-chip systems.
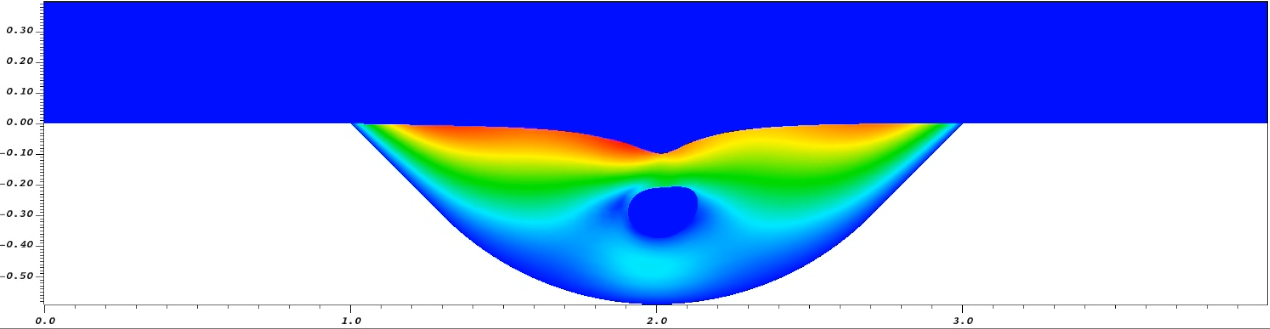
More recently, we investigated the mass and force transmission in OoCs by computer simulations. The aim is to predict how much mechanical stress and chemical factors penetrate the ECM to reach the cells. This requires an accurate account of the fluid-solid mechanics of the hydrogel matrix. Our model predicts, for example, that cells experience stresses on the order of several milli-pascals under typical flow conditions, coinciding with the optimal stress for liver regeneration measured in a liver-on-chip device. The movie below shows an example of how the perfusion in the channel (blue) moves and deforms a cell embedded inside a hydrogel matrix, with the color indicating the speed of the interstitial flow inside the gel:
We have also discovered "stress ribbons" as a mechanism for mechanical cross-talk among neighbors in a cell assembly. The image below shows the vicinity of a 7-cell assembly inside the same gel domain as shown above. The color contours show the magnitude of the von Mises stress computed from the gel elastic stress tensor:
In collaboration with experimentalists, we are currently investigating the flow and stress distribution inside an in vitro model of a liver sinusoid. Our aim is to understand how the mechanical stresses affect hepatocyte function and regeneration inside the space of Disse.